Principle, Types, Method, Uses
The hemagglutination assay stands as one of the most valuable diagnostic tools in veterinary medicine. This simple yet powerful laboratory technique helps veterinarians detect and identify various infectious agents affecting animals. The hemagglutination assay is used to detect and quantify viruses, bacteria, or antibodies based on their ability to agglutinate red blood cells (RBCs). From diagnosing avian influenza in poultry to detecting equine infectious anemia in horses, hemagglutination testing plays a crucial role in maintaining animal health and preventing disease outbreaks.
The hemagglutination assay (HA) was developed in the early 1940s by American virologist George Hirst. It is primarily used in virology and immunology to determine the presence and concentration of viruses, such as the influenza virus, and to evaluate immune responses. Understanding this diagnostic method is essential for veterinary professionals, animal health workers, and livestock farmers who need rapid, cost-effective disease detection solutions.
What is Hemagglutination Assay?
Hemagglutination assay is a laboratory test that detects the clumping (agglutination) of red blood cells. This clumping occurs when certain viruses, bacteria, or antibodies bind to receptors on the surface of red blood cells, causing them to stick together and form visible clusters.
In veterinary practice, this test serves as a frontline diagnostic tool because it requires minimal equipment, provides rapid results, and can be performed in field conditions. The visual nature of the test makes it particularly useful in resource-limited settings where sophisticated laboratory equipment may not be available.
Importance in Veterinary Disease Diagnosis
Why Hemagglutination Matters in Animal Health
The hemagglutination assay holds immense importance in veterinary diagnostics for several compelling reasons:
Early Disease Detection: The test identifies infections before animals show clinical symptoms, allowing for timely intervention and treatment. Early detection prevents disease spread within herds and flocks.
Cost-Effective Screening: Compared to molecular techniques like PCR, hemagglutination testing requires less expensive reagents and equipment, making it accessible for routine screening in veterinary clinics and farms.
Rapid Results: Most hemagglutination tests provide results within 30 minutes to 2 hours, enabling quick decision-making during disease outbreaks.
Surveillance Programs: The test plays a vital role in monitoring disease prevalence in animal populations, helping authorities implement effective control measures.
Trade and Export Requirements: Many countries require hemagglutination test results before importing livestock, poultry, or animal products, making it essential for international animal trade.
Principle of Hemagglutination Assay
The Science Behind the Test
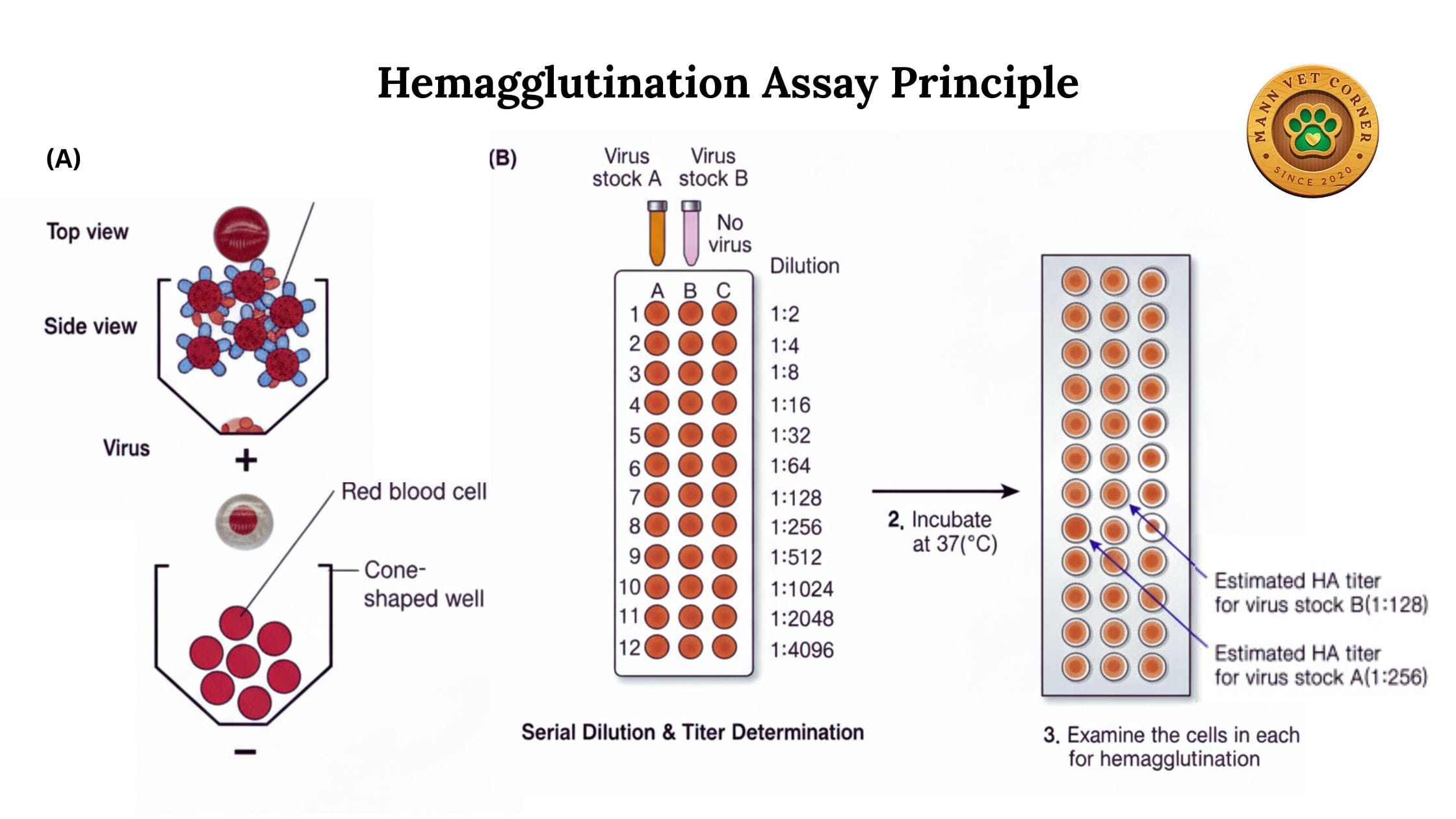
The fundamental principle of hemagglutination involves the interaction between red blood cells and agglutinating agents. Here’s how it works:
Surface Receptors: Red blood cells possess specific surface receptors, primarily sialic acid residues, which serve as binding sites.
Viral Attachment: Many viruses have surface proteins (hemagglutinins) that naturally bind to these receptors. When virus particles encounter red blood cells, they attach to multiple cells simultaneously.
Cross-Linking: A single virus particle can bind to receptors on different red blood cells, creating cross-links. This cross-linking causes the cells to clump together, forming visible aggregates.
Lattice Formation: As more virus particles interact with more red blood cells, a lattice network forms, creating a characteristic pattern that spreads across the bottom of a test well or tube.
Visual Detection: In a positive test, the agglutinated cells form a diffuse mat or shield pattern. In a negative test, unagglutinated cells settle into a compact button at the bottom of the well.
Antibody-Mediated Hemagglutination
When testing for antibodies rather than viruses, the principle involves two stages:
- Primary Binding: Antibodies in the test serum bind to viral antigens
- Inhibition: These antibody-virus complexes cannot bind to red blood cells, preventing hemagglutination
- Interpretation: The presence of antibodies is indicated by the absence of agglutination (a paradoxical but important concept)
Types of Hemagglutination Assays
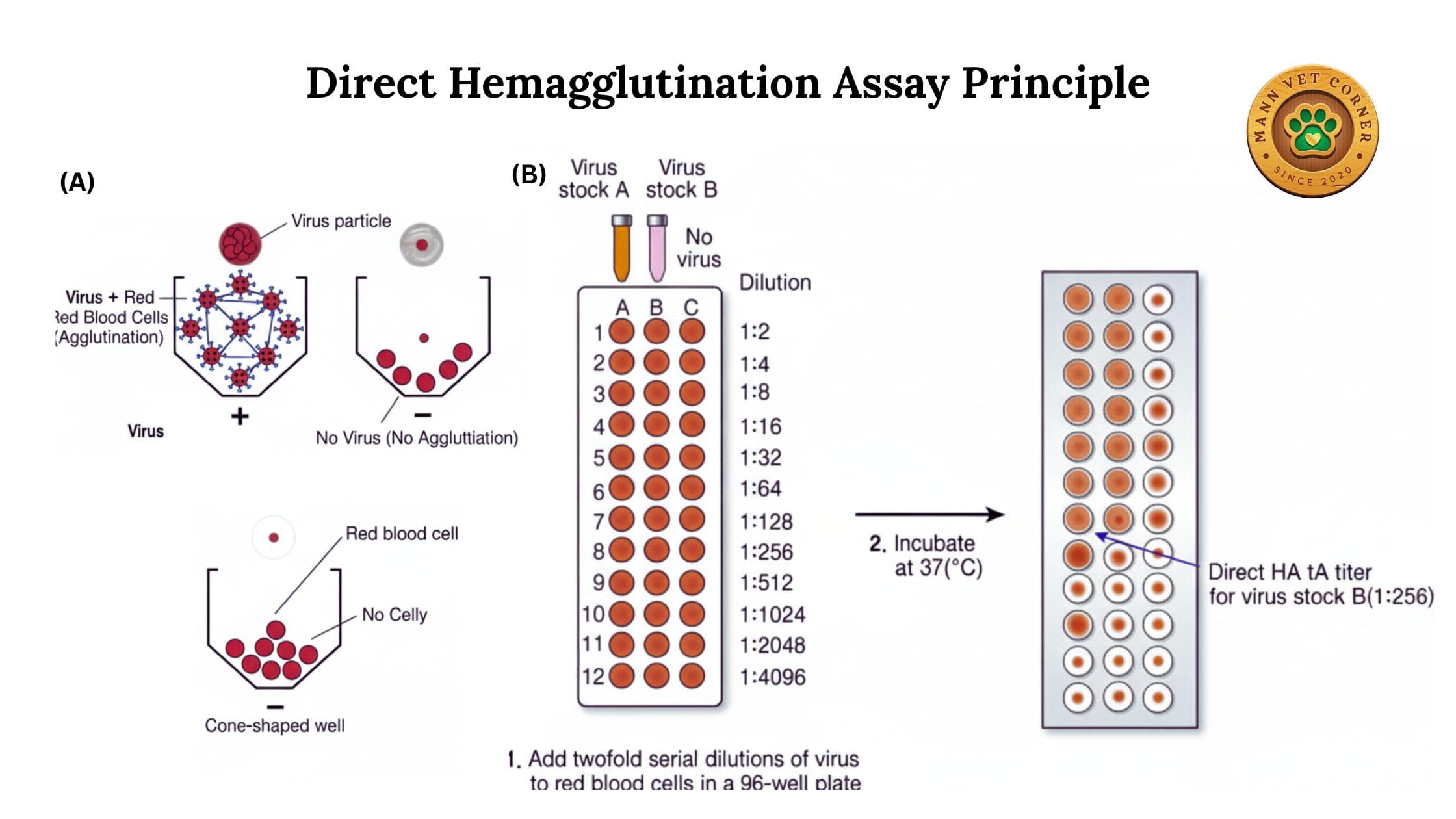
1. Direct Hemagglutination Assay (HA)
Direct hemagglutination detects the presence of viral particles or other agglutinating agents directly in a sample.
Procedure: Test samples are mixed with red blood cells, and agglutination indicates the presence of virus or other hemagglutinating agents.
Veterinary Applications:
- Detecting avian influenza virus in poultry samples
- Identifying Newcastle disease virus in chickens
- Screening for infectious bronchitis virus in birds
- Testing for canine parvovirus in dogs
Advantages: Quick, simple, and requires minimal preparation of samples.
Limitations: Cannot differentiate between different strains or determine antibody levels.
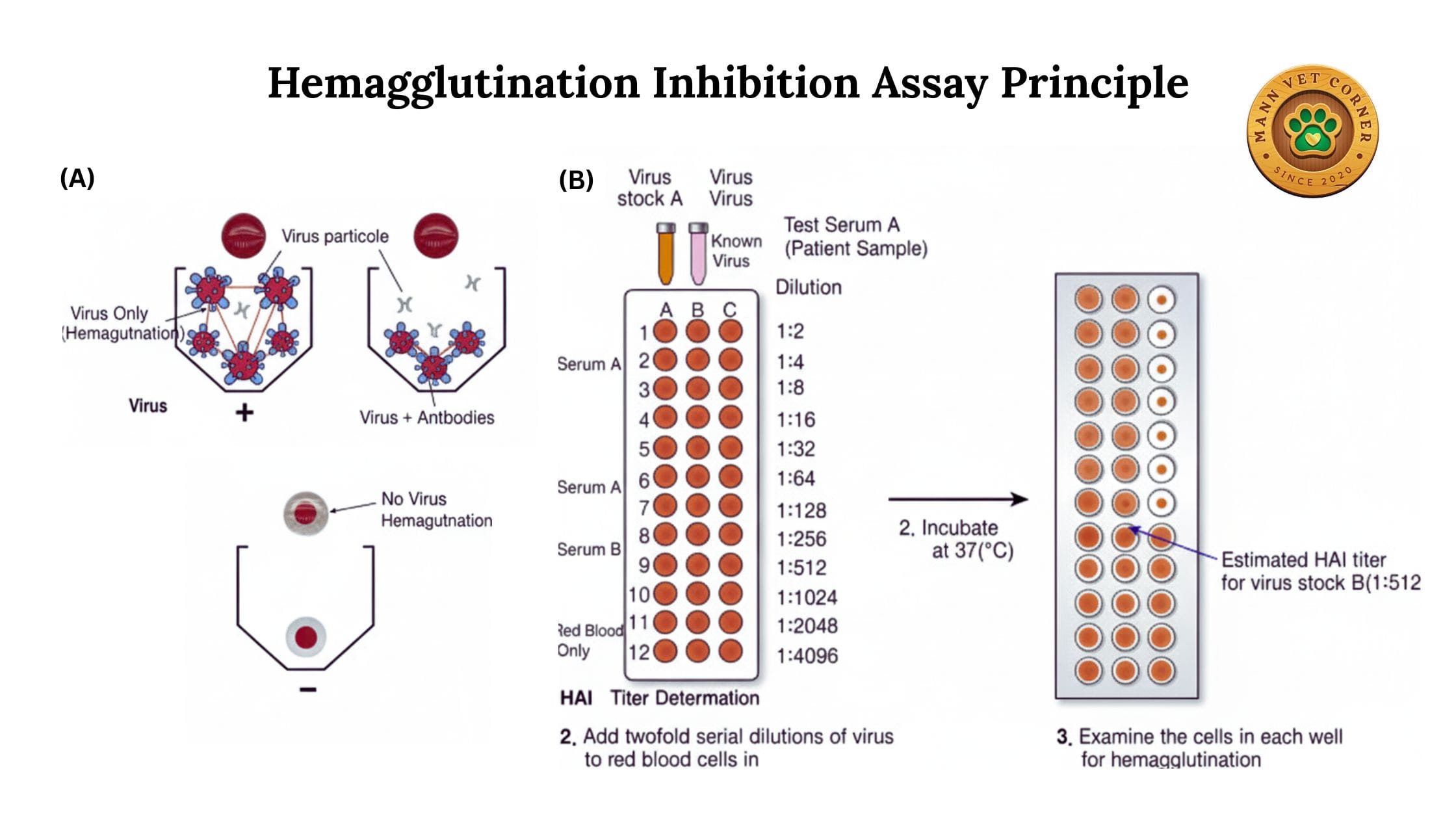
2. Hemagglutination Inhibition Test (HI)
The HI test is the most widely used variant in veterinary diagnostics. It measures antibodies in animal serum by their ability to inhibit viral hemagglutination.
Procedure: Serial dilutions of serum are mixed with a known amount of virus before adding red blood cells. Antibodies in the serum neutralize the virus, preventing hemagglutination.
Veterinary Applications:
- Monitoring vaccination responses in livestock and poultry
- Detecting equine influenza antibodies in horses
- Screening for swine influenza in pigs
- Confirming avian influenza exposure in birds
- Testing for rabies antibodies in vaccinated animals
Advantages: Quantitative results (antibody titers), highly specific, and standardized protocols available.
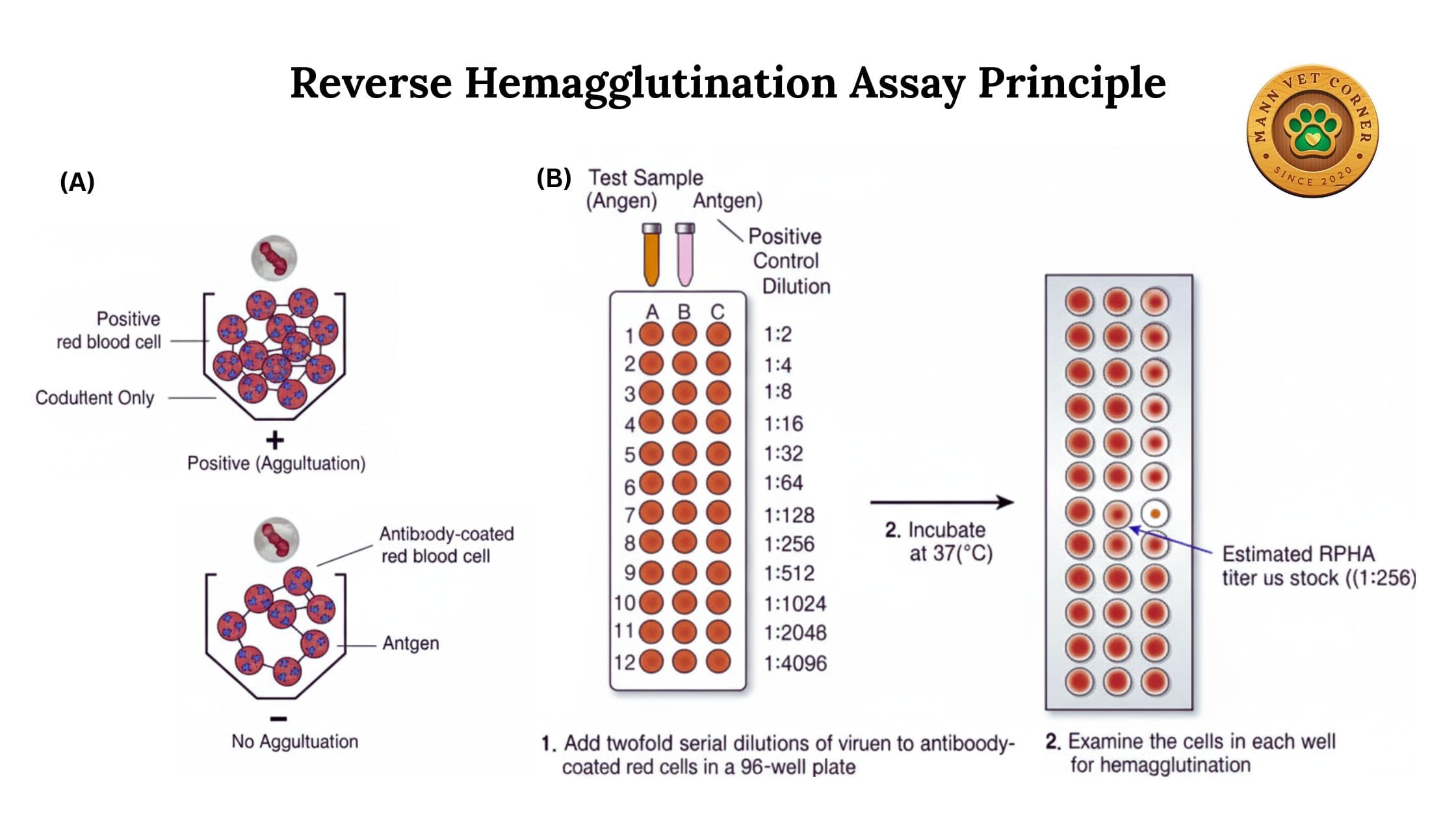
3. Reverse Passive Hemagglutination (RPHA)
In this variant, red blood cells are artificially coated with antigens or antibodies, expanding the test’s applications.
Procedure: Red blood cells are sensitized with specific antigens. When mixed with serum containing corresponding antibodies, agglutination occurs.
Veterinary Applications:
- Detecting antibodies against bacteria like Brucella or Leptospira
- Screening for autoimmune conditions in companion animals
- Identifying antibodies in diseases that don’t naturally cause hemagglutination
Advantages: Extends hemagglutination testing to non-hemagglutinating pathogens.
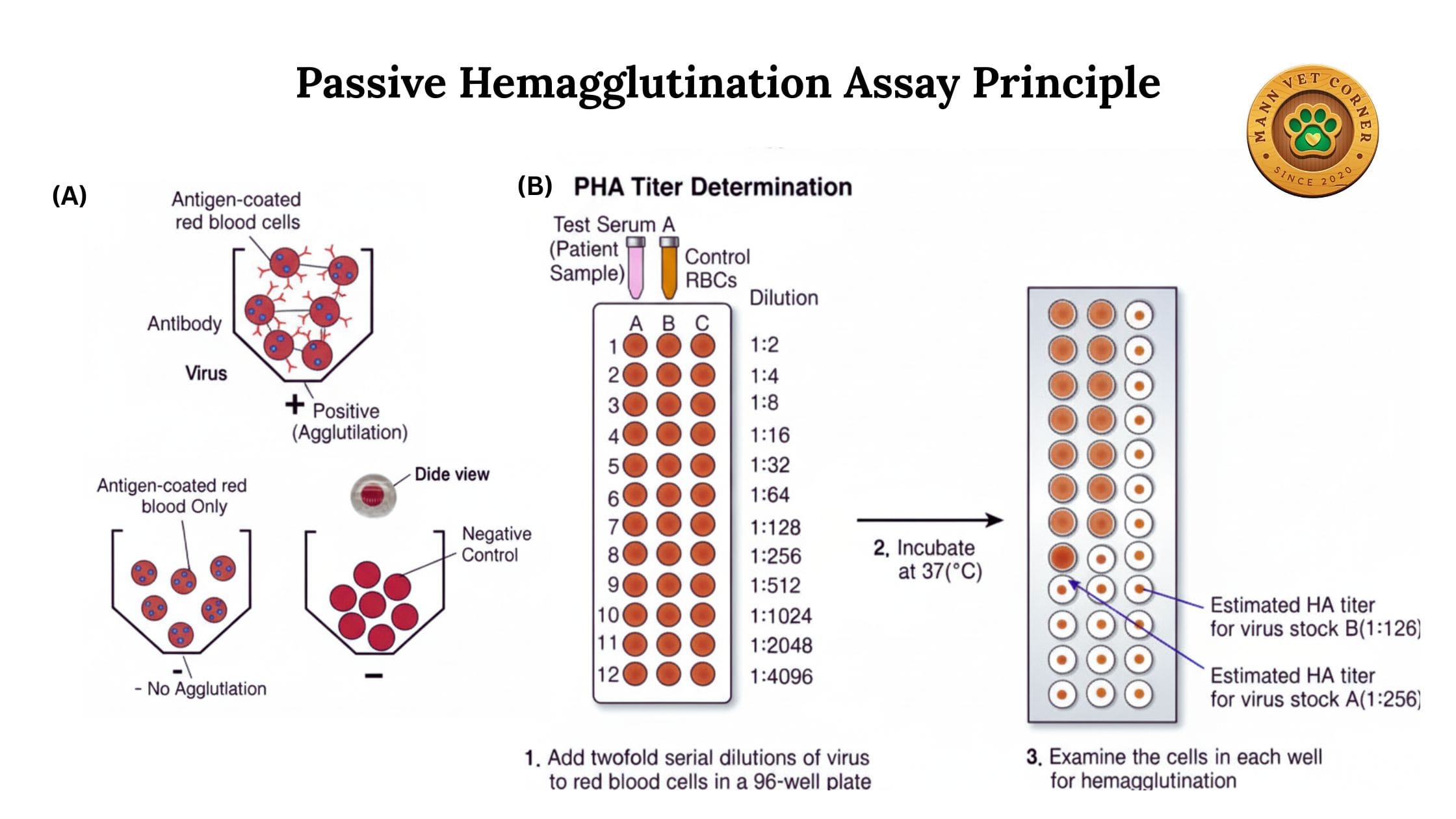
4. Passive Hemagglutination (PHA)
Similar to RPHA but used primarily for detecting antibodies against bacterial or parasitic antigens.
Veterinary Applications:
- Diagnosing toxoplasmosis in cats and sheep
- Detecting trichomoniasis in cattle
- Screening for various bacterial infections
Advantages: offers high sensitivity, simplicity, and cost-effectiveness for detecting specific antibodies or antigens in veterinary diagnostics.
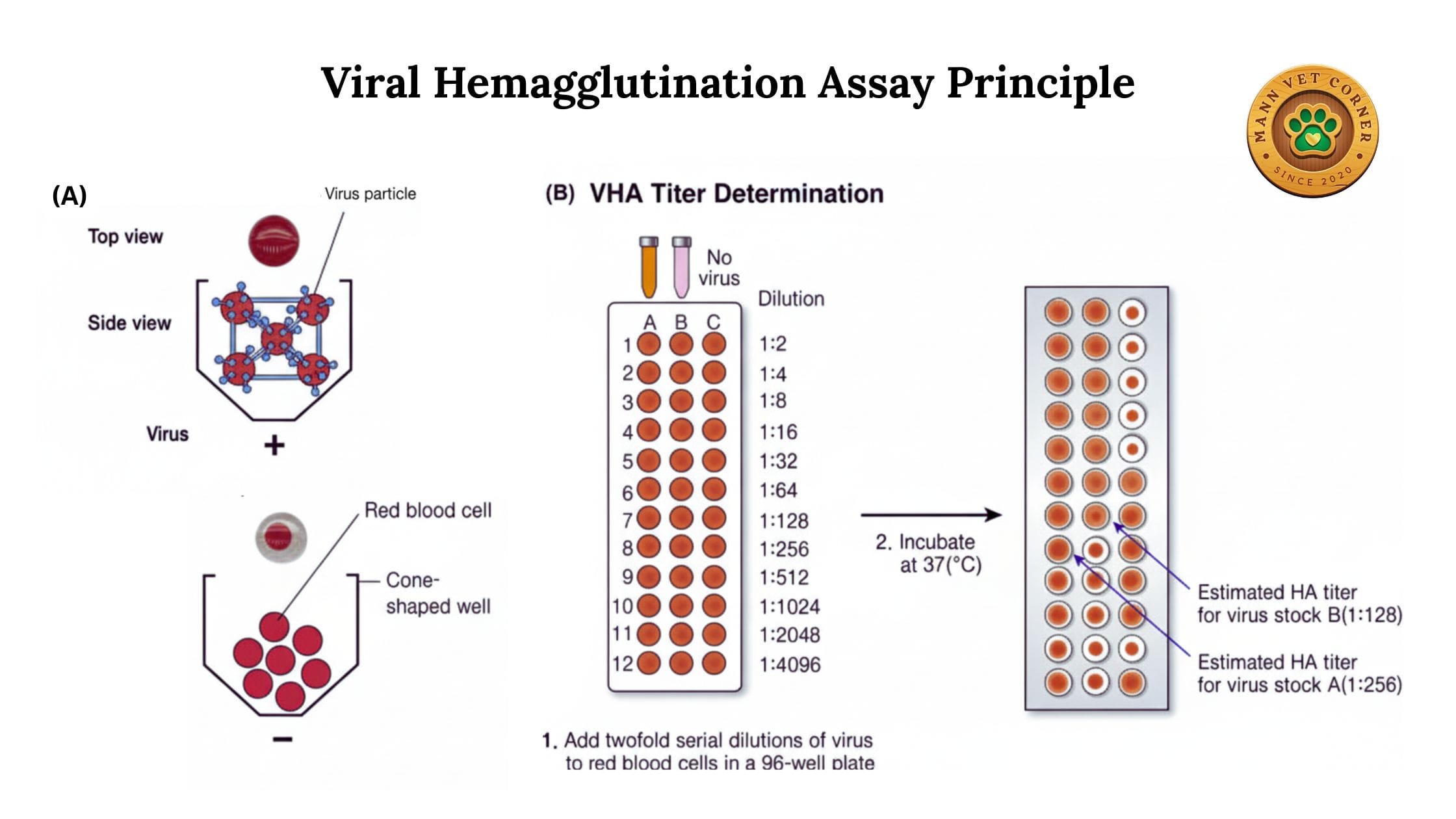
5. Viral Hemagglutination Assay
Specifically designed to quantify the amount of virus in a sample by determining the highest dilution that still causes hemagglutination.
Veterinary Applications:
- Determining viral load in research settings
- Standardizing vaccine preparations
- Quality control of viral vaccines for animals
Advantages: enables rapid, cost-effective detection and quantification of viruses based on their ability to agglutinate red blood cells.
Method and Procedure
Materials Required
Red Blood Cells:
- Chicken, sheep, guinea pig, or horse red blood cells (depending on the virus)
- Fresh cells (less than one week old) work best
- Cells should be washed and prepared as 0.5-1% suspension
Equipment:
- Microtiter plates (96-well V-bottom or U-bottom)
- Micropipettes and multichannel pipettes
- Phosphate buffered saline (PBS)
- Test tubes for dilutions
- Refrigerated centrifuge
Samples:
- Serum samples from animals (for antibody detection)
- Viral suspensions or clinical samples (for direct HA)
- Positive and negative controls
Step-by-Step Procedure for Hemagglutination Inhibition Test
Sample Collection and Preparation
- Collect blood samples from animals using proper restraint and sterile technique
- Allow blood to clot at room temperature for 30-60 minutes
- Centrifuge samples at 3000 rpm for 10 minutes
- Carefully remove serum and transfer to clean tubes
- Heat-inactivate serum at 56°C for 30 minutes to destroy complement
- Store samples at -20°C if not testing immediately
Red Blood Cell Preparation
- Collect fresh blood in anticoagulant (citrate or EDTA)
- Wash cells three times with PBS
- Prepare 0.5% to 1% suspension in PBS
- Use cells within 5-7 days of collection
- Store at 4°C between uses
Viral Antigen Preparation
- Dilute virus to contain 4 hemagglutinating units (4 HAU)
- Perform preliminary titration to determine correct dilution
- Prepare fresh on the day of testing
- Keep viral antigen on ice during the procedure
Test Execution
- Serum Dilution: Add 25 μL PBS to all wells in rows. Add 25 μL serum to first well, perform serial two-fold dilutions across the row (1:2 to 1:2048)
- Virus Addition: Add 25 μL of viral antigen (4 HAU) to all wells. Mix gently by tapping the plate
- Incubation: Incubate at room temperature for 30-60 minutes to allow antibody-virus interaction
- RBC Addition: Add 50 μL of 0.5% red blood cell suspension to all wells. Mix gently
- Final Incubation: Incubate at room temperature or 4°C for 30-60 minutes until control wells show clear patterns
- Reading Results: Tilt the plate to 45 degrees. Positive wells (antibody present) show streaming or button formation. Negative wells show shield or umbrella pattern
Quality Control
Include the following controls in every test:
- Positive Serum Control: Known positive sample to verify test performance
- Negative Serum Control: Known negative sample to confirm specificity
- RBC Control: Red blood cells in PBS only (should show button)
- Virus Control: Virus with RBCs without serum (should show complete hemagglutination)
Interpretation of Results
Antibody Titer: The reciprocal of the highest serum dilution that completely inhibits hemagglutination. For example, if the last well showing inhibition is 1:256, the titer is 256.
Protective Levels: Each disease has specific protective titer levels:
- Avian influenza: ≥16 to ≥32 (varies by strain)
- Newcastle disease: ≥16 to ≥128
- Equine influenza: ≥40 to ≥80
Interpretation Guidelines:
- Negative: Titer less than 8 (no antibodies detected)
- Low positive: Titer 8-32 (possible early infection or waning immunity)
- Moderate positive: Titer 64-128 (adequate immune response)
- High positive: Titer ≥256 (recent infection or strong vaccination response)
Uses in Veterinary and Animal Disease Diagnosis
Poultry Diseases
Avian Influenza: The HI test remains the gold standard for AI surveillance programs worldwide. It detects antibodies against different AI subtypes, helping veterinarians monitor flock immunity and identify exposed birds.
Newcastle Disease: Regular HI testing ensures vaccination programs are effective. Farmers can adjust vaccination schedules based on antibody titers, preventing outbreaks in commercial poultry operations.
Infectious Bronchitis: The test helps differentiate between vaccine strains and field strains, crucial for understanding disease patterns in layer and broiler flocks.
Avian Reovirus: HI testing identifies infected flocks and monitors the effectiveness of vaccination programs for tenosynovitis and malabsorption syndrome.
Equine Diseases
Equine Influenza: Pre-export testing, competition screening, and monitoring vaccination efficacy all rely on HI assays. Horses must maintain specific antibody titers for international travel and competition.
Equine Infectious Anemia: The coggins test, a form of hemagglutination assay, is mandatory in many jurisdictions for horse movement and sale.
Equine Viral Arteritis: HI testing helps distinguish between vaccinated and naturally infected horses, important for breeding programs.
Swine Diseases
Swine Influenza: Monitoring influenza antibodies in pig herds helps prevent zoonotic transmission and maintain herd health. Commercial pig operations routinely test breeding stock.
Porcine Reproductive and Respiratory Syndrome (PRRS): Modified hemagglutination tests help detect antibodies in breeding herds.
Ruminant Diseases
Bluetongue Virus: In sheep and cattle, HI testing supports surveillance programs and helps evaluate vaccine efficacy in endemic regions.
Epizootic Hemorrhagic Disease: Deer and cattle populations are monitored using HI assays to track disease spread.
Foot-and-Mouth Disease: Although ELISA has largely replaced it, HI testing historically played a major role in FMD diagnosis and continues to be used in some regions.
Companion Animals
Canine Distemper: HI tests measure antibody responses to vaccination in dogs, ensuring adequate protection.
Canine Parvovirus: Testing maternal antibody levels helps veterinarians determine optimal vaccination timing in puppies.
Feline Panleukopenia: Similar to canine parvovirus, HI testing guides vaccination protocols in catteries and shelters.
Wildlife Disease Surveillance
Rabies: HI testing measures antibody responses in vaccinated wildlife populations, crucial for oral rabies vaccination programs targeting foxes, raccoons, and skunks.
West Nile Virus: Surveillance in wild bird populations uses HI assays to track virus spread and identify high-risk areas.
Advantages of Hemagglutination Assay
- Simplicity: Does not require sophisticated equipment or extensive technical training
- Cost-Effectiveness: Reagents are inexpensive compared to molecular or ELISA tests
- Rapid Results: Most tests completed within 1-2 hours
- Quantitative: Provides antibody titers, not just positive/negative results
- Standardization: Well-established protocols approved by international organizations (OIE, WHO)
- Field Application: Can be performed in basic laboratory settings or even field conditions
- Large-Scale Screening: Multiple samples tested simultaneously in microtiter format
- Historical Data: Decades of reference data available for comparison
Limitations and Challenges
- Species-Specific RBCs: Different viruses require specific red blood cell types, requiring maintenance of multiple cell sources
- Subjective Reading: Results interpretation can be subjective, requiring experienced technicians
- Non-Specific Reactions: Some samples contain non-specific inhibitors causing false results
- Sensitivity: May be less sensitive than molecular methods for early infection detection
- Cross-Reactivity: Antibodies may cross-react between related viruses, complicating interpretation
- Time-Sensitive Reagents: Red blood cells have limited shelf life, requiring fresh preparation
- Prozone Effect: Very high antibody levels may cause false negative results without proper dilution
Tips for Accurate Testing
- Use Fresh Reagents: Always prepare red blood cells fresh and use within recommended timeframes
- Proper Controls: Include comprehensive positive and negative controls in every test run
- Consistent Technique: Maintain consistent pipetting, mixing, and incubation times
- Temperature Control: Follow specified incubation temperatures precisely
- Sample Quality: Ensure serum samples are non-hemolyzed and properly heat-inactivated
- Training: Ensure technicians receive proper training in reading hemagglutination patterns
- Documentation: Maintain detailed records of results, controls, and reagent lot numbers
- Validation: Periodically validate results against reference laboratories
Future Perspectives
While molecular techniques continue advancing, hemagglutination assays remain relevant in veterinary diagnostics. Emerging developments include:
Automated Reading Systems: Digital imaging and artificial intelligence systems are being developed to standardize result interpretation and eliminate reader bias.
Miniaturization: Microfluidic devices may allow point-of-care hemagglutination testing with smaller sample volumes.
Enhanced Sensitivity: Modified protocols and reagents are improving test sensitivity to match molecular methods.
Combination Testing: Integration with other rapid tests for comprehensive disease screening panels.
Conclusion
The hemagglutination assay continues to serve as a cornerstone of veterinary diagnostic medicine. Its simplicity, reliability, and cost-effectiveness make it indispensable for disease surveillance, vaccination monitoring, and outbreak investigation in animal populations. Despite the emergence of advanced molecular techniques, hemagglutination testing maintains its position as a practical, accessible tool for veterinarians worldwide.
Understanding the principles, types, methods, and applications of hemagglutination assays empowers veterinary professionals to make informed diagnostic decisions, implement effective disease control measures, and safeguard animal health. As technology advances, traditional hemagglutination testing will likely evolve and integrate with modern approaches, ensuring its continued relevance in protecting animal welfare and public health.
Frequently Asked Questions (FAQs)
1. What is the difference between hemagglutination and hemagglutination inhibition?
Hemagglutination (HA) detects viruses or other agents that cause red blood cells to clump together. Hemagglutination inhibition (HI) measures antibodies in serum by their ability to prevent viral hemagglutination. HA is used for direct virus detection, while HI tests for immunity or previous exposure by measuring antibody levels.
2. Which red blood cells are best for veterinary hemagglutination testing?
The choice depends on the virus being tested. Chicken red blood cells work well for avian influenza and Newcastle disease. Guinea pig cells are preferred for certain mammalian viruses. Sheep or horse cells are used for specific equine viruses. Always consult reference protocols for the specific pathogen being tested.
3. How long does a hemagglutination assay take to complete?
A complete hemagglutination inhibition test typically takes 2-3 hours from start to finish. This includes serum dilution (30 minutes), virus-antibody incubation (30-60 minutes), red blood cell incubation (30-60 minutes), and result reading (15 minutes). Direct hemagglutination tests are faster, often completed within 1 hour.
4. Can hemagglutination tests differentiate between vaccinated and infected animals?
Standard HI tests cannot distinguish between antibodies from vaccination versus natural infection, as both produce antibodies against the same viral antigens. However, testing paired samples taken 2-3 weeks apart can show rising titers indicative of active infection. Some modified tests using specific antigens can make this distinction.
5. What is a protective antibody titer in hemagglutination testing?
Protective titers vary by disease and species. For avian influenza in chickens, titers above 16-32 suggest adequate immunity. Newcastle disease requires titers above 16-128 depending on the strain. Equine influenza protection typically requires titers above 40-80. Always refer to specific guidelines for the disease in question.
6. Why must serum be heat-inactivated before hemagglutination testing?
Heat inactivation at 56°C for 30 minutes destroys complement proteins in serum that might cause non-specific hemagglutination or interfere with the test. This process eliminates false positive results and improves test accuracy without affecting antibody activity.
7. How often should animals be tested using hemagglutination assays?
Testing frequency depends on the purpose. For routine vaccination monitoring, test 2-3 weeks post-vaccination and then annually. In high-risk areas or during outbreaks, monthly testing may be necessary. Export requirements typically mandate testing within 21-30 days before shipment. Breeding animals may require quarterly testing.
8. Can hemagglutination tests be performed on-farm or require a laboratory?
Basic hemagglutination tests can be performed in simple on-farm laboratories with minimal equipment (microtiter plates, pipettes, and refrigeration). However, proper training, quality control, and reagent preparation are essential. For official diagnostic purposes or certification, testing at accredited veterinary laboratories is usually required.
9. What causes false positive results in hemagglutination testing?
False positives can occur from lipemic (fatty) serum, bacterial contamination, hemolyzed samples, or presence of non-specific inhibitors in serum. Improper heat inactivation, incorrect red blood cell concentration, or contaminated reagents may also cause false results. Proper sample handling and quality controls minimize these issues.
10. Is hemagglutination testing still relevant with modern PCR available?
Yes, hemagglutination testing remains highly relevant. While PCR detects active infections more sensitively, HI testing measures immune status, vaccination responses, and herd immunity levels that PCR cannot assess. The cost-effectiveness and simplicity of HI tests make them ideal for large-scale surveillance and routine monitoring, especially in resource-limited settings.